Simplifying SaaS GxP Assessments: From Decisions to Scalable Execution
Determining whether a SaaS system requires GxP validation shouldn’t involve 100+ questions across dozens of pages. Yet many life sciences teams still rely on long, overlapping questionnaires that slow decisions and add unnecessary cost.
For small and emerging pharma, this approach is inefficient—and avoidable.
At Rosenberg Life Science Consulting, I help teams simplify GxP decisions while maintaining inspection-ready compliance.
The 10 Questions That Matter Most for GxP Determination
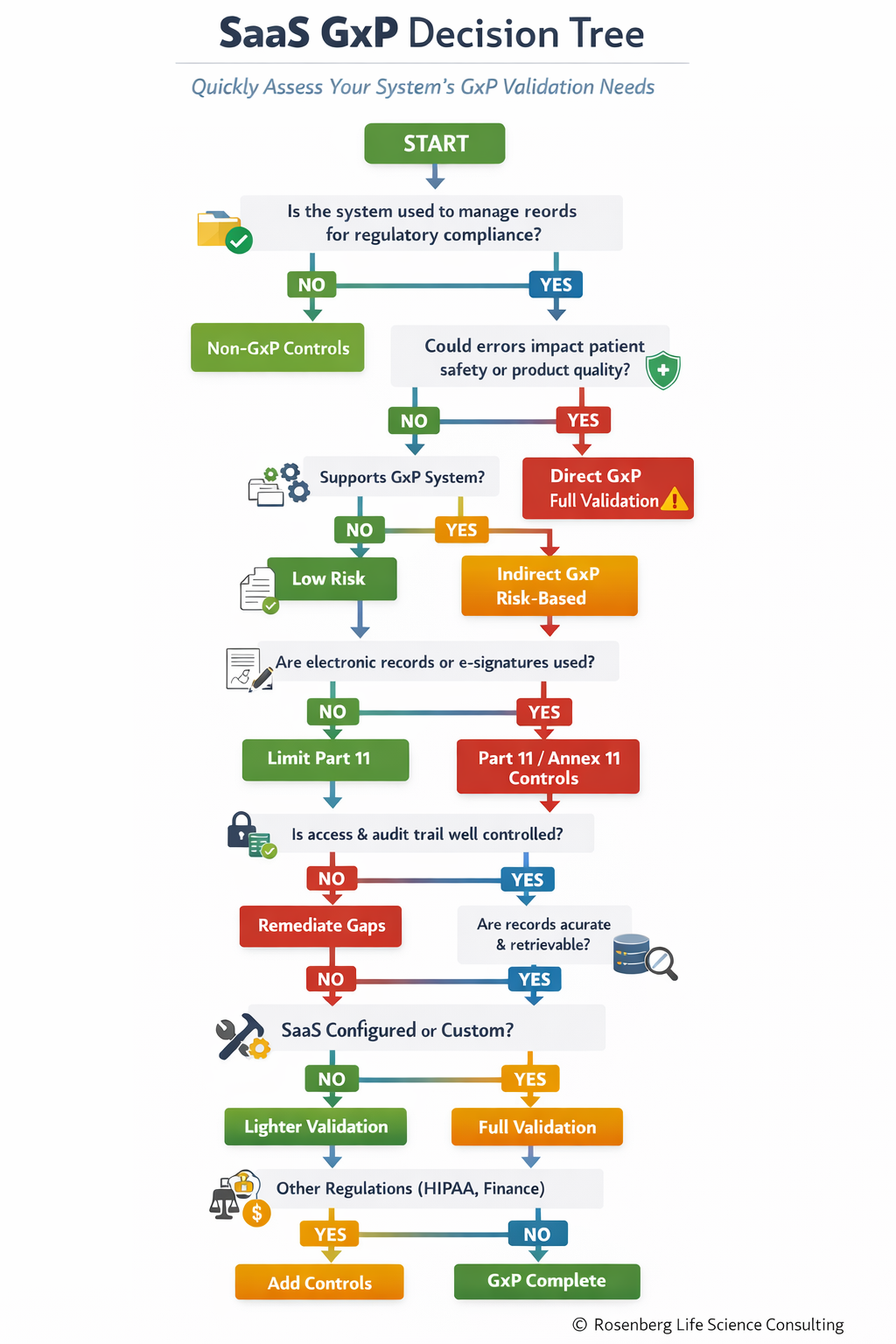
You don’t need an exhaustive questionnaire to determine GxP impact. These 10 base questions drive every downstream compliance decision:
Is the system used for regulatory records?
Could errors impact patient safety or product quality?
Does it support another GxP-critical system?
Are electronic records or signatures used?
Are access controls and audit trails required?
Can data be accurately retrieved?
Is the system standard, configured, or custom?
Does the vendor maintain a quality system?
Are there additional regulatory overlays (HIPAA, privacy, financial)?
Answering these questions allows teams to confidently determine:
Full GxP validation
Risk-based validation
Non-GxP controls
Visualizing GxP Decisions: One-Page Decision Tree
To make these decisions practical and repeatable, I created a one-page GxP decision tree that turns the 10 questions into a simple, visual workflow.
📊 View the GxP Decision Tree Below
SaaS GXP Decision Tree
How teams use it:
Align quickly with QA and Regulatory
Eliminate unnecessary documentation
Standardize future system assessments
Support vendor selection and system upgrades
Tip: Many teams keep this decision tree as part of their governance or validation playbook.
From GxP Decision to Execution
Once a system is deemed GxP-relevant, execution is where many programs struggle:
Validation treated as documentation—not an operating model
Quality decisions disconnected from delivery timelines
Controls were added late instead of designed in early
Lost traceability between requirements, risk, and testing
Strong execution focuses on:
Clear ownership from day one
Fit-for-purpose, inspection-ready controls
End-to-end traceability across the system lifecycle
With structured program leadership—and increasingly AI-enabled tools—teams can surface risk earlier, reduce rework, and scale execution predictably.
How Rosenberg Life Science Consulting Helps
Rosenberg Life Science Consulting partners with life sciences teams to:
Simplify SaaS GxP assessments
Establish defensible validation strategies
Lead execution across clinical, safety, quality, and enterprise systems
Deliver scalable, inspection-ready outcomes